So2 Molecular Geometry And Polarity
Sulfur dioxide (SO2) is polar in nature. The electronegativity deviation between sulfur (2.58) and oxygen (3.44) atoms makes information technology a polar molecule. In addition, due to the presence of unbonded electrons on the sulfur and oxygen atoms, SO2 has a bent shape.
| Name of molecule | Sulfur dioxide |
| Bail Angles | 119 degrees |
| Molecular Geometry of SO2 | Trigonal planar |
| Hybridization of SO2 | sptwo hybridization |
| No Valence Electrons in the molecule | xviii |
| SO2 Polar or Nonpolar | Polar |
| The dipole moment of SO2 | 1.61 debye |
The polarity of the SO2 molecule
In SO2 molecules, the electronegative potential of oxygen is higher than that of sulfur.
As a upshot, oxygen exerts a stronger pull on sulfur dioxide'southward covalent bonds.
The side of the molecule containing both oxygen atoms is somewhat negatively charged.
whereas the portion that has the sulfur cantlet has a slightly positive charge.
This makes SO2 a polar molecule.
In addition, the unbonded electrons on the sulfur and oxygen create repulsion betwixt atoms.
This is some other crusade of the polarity of the sulfur dioxide molecule.
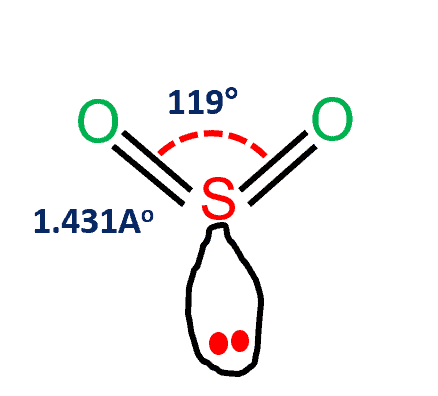
SO2 Molecular Geometry
The SO2 molecular geometry is bent, with a bond angle of 120°. The distribution of charges on the key atom is asymmetrical. The part of the molecule containing both oxygen atoms is somewhat negatively charged. The part containing the sulfur atom, on the other hand, has a slightly positive charge. In that location is also a lonely pair of electrons on the sulfur besides.
Betwixt sulfur and each oxygen atom, ane sigma and one pi bond are formed. To reduce repulsions, the double bonds and the solitary pair are separated every bit much as possible, and and then the molecule is aptitude.
What is sulfur dioxide (And then2)?
Sulfur Dioxide (American English language), also known every bit Sulfur Dioxide (Democracy English), is a bail between sulfur and oxygen atoms. Information technology's a colorless, poisonous, inorganic gas with a noxious aroma like to nitric acid. Volcanic activity releases this gas naturally.
When dissolved in water, it produces a weak acid solution.
Sulfur dioxide is a key precursor of sulfuric acid and is found at modest levels in the surround.
Molar Mass of Sulfur dioxide
The molar mass of Due south is 32.066 g/mol.
Oii molar mass = 16.00 10 2 = 32.00 g/mol.
Molar mass of And so2 = 64.066 one thousand/mol
Primal Points
- SO2 Lewis structure would comprise ii atoms of oxygen (O) and one sulfur cantlet. The number of valence electrons in both Southward and O atoms is vi. The full number of SO2 valence electrons is 12.
- The electron geometry of SO2 is formed in the shape of a trigonal planner.
- The three pairs of bonding electrons are arranged in the aeroplane at an angle of 120 degrees.
- The sulfur valence electron is equal to 6.
- valence electrons of oxygen = half-dozen (In that location are 2 oxygen atoms in the compound.)
Production of sulfur dioxide
Sulfur or atomic number 26 pyrites are burnt over the air to produce SO2.
Due south + O2 (gas) —> SOtwo (gas)
4FeS2 + 11O2 –> 2FeiiO3 (solid) + 8SO2 (gas)
Similarities between Sulfur and Oxygen atoms
- Both O and S accept the same outer electric configuration of ns2 and np4.
- O and S are usually divalent.
- Both showroom an allopatric form.
- O and S are not-metals.
- In reaction with metals, both react with the oxidation state of-2.
- While reacting with nonmetals, both form covalent compounds, for case, Water, H2S, CO2, and CStwo.
Dissimilarities between oxygen and sulfur
| Oxygen | Sulfur |
| Two allotropic forms | three allotropic forms |
| Gas at ordinary temperature | Solid at ordinary temperature |
| Sparingly soluble in water | Not soluble in water |
| Helps in combustion | Combustible itself |
| Paramagnetic in nature | diamagnetic in nature |
| Does non react with water | When steam is passed through boiling sulfur, a fiddling hydrogen sulfide and sulfur dioxide is formed. |
| Does not react with acids | It is readily oxidized past concentrated sulfuric acrid or nitric acrid. |
Sulfur dioxide Effects on Humans
Sulfur dioxide is a poisonous gas that is hazardous to human wellness.
Information technology can irritate the eyes, nose, pharynx, and lungs' skin and mucous membranes.
Its excessive amounts might irritate and induce inflammation in the respiratory arrangement.
Sulfur dioxide emissions in the air can lead to the cosmos of other sulfur oxides (SOx).
SOx can react with other compounds in the atmosphere to form small particles.
These small particles may penetrate deeply into the lungs, and their sufficient quantity can contribute to health bug.
Summary
- The molecular geometry of sulfur dioxide is a aptitude shape.
- The sulfur to oxygen ratio in sulfur dioxide is i:2.
- The sulfur dioxide molecule has ii double bonds betwixt the sulfur atom and oxygen atoms.
- There are v lone pairs of electrons in the molecule of SO2.
- The molar mass of sulfur dioxide is 64.066 m/mol.
- SO2 gives a weak acid solution when dissolved in water.
SiOii Lewis Structure
North2O Lewis Structure| Laughing Gas
HCN Lewis Structure
Co2 Polar or Nonpolar
Is HCN Polar or Nonpolar?
NH3 Oxidation Number
Is HCl Polar or Nonpolar?
Oftentimes Asked Questions (FAQs)
Some of the frequently asked questions are given below. If you take whatsoever questions, feel complimentary to annotate or send an email to hello.whatsinsight@gmail.com
1. How many lone pairs of electrons are nowadays in the SO2 Lewis construction?
There are two double bonds between the sulfur and oxygen atoms in the sulfur dioxide molecular geometry. Each sulfur atom has one lonely pair, while each oxygen atom has ii.
2. What is the biggest source of SO2?
Burning fossil fuels is the largest source of SO2 in the atmosphere.
3. What is sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
4. Is BF3 polar or nonpolar?
BF3 is a non-polar chemical compound. In BFiii, the fundamental boron atom has sptwo hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the boron-fluorine bonds are all 120 degrees apart, any cyberspace dipole in that aeroplane is canceled out. Even if each B-F bond is polar, the net dipole moment is nil because adding the bond vectors cancels everything out.
Check the full article "Is BF3 polar or nonpolar?".
5. What is Sulfur hexafluoride?
Sulfur hexafluoride (SF6) is a not-toxic gas that is used in a diversity of applications due to its inert properties. While SF6 is not-toxic when used properly, toxic byproducts tin be produced during electrical discharges within SF6-filled equipment, posing a threat to the health of workers who come into contact with them.
6. What is Sulfur trioxide?
SO3 (sulfur trioxide) is a chemic compound. It is bachelor in three forms: gaseous monomer, crystalline trimer, and solid polymer. It is solid at just below room temperature and has a relatively narrow liquid range. Gaseous SO3 is the principal forerunner to acid rain.
7. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bail, nitrogen is more than electronegative than hydrogen. This unequal distribution of charges amongst both nitrogen and hydrogen atoms makes NH3 a polar molecule.
8. CH4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule.
9. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the 4 chemical bonds betwixt silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the deviation in electronegativity between the anion and the cation is betwixt 0.4 and i.7, such bonds occur.
Check the full article "Is SiCl4 polar or nonpolar?".
10. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may flare-up violently if exposed to burn down or heat for a lengthy menstruation of fourth dimension.
So2 ionic or covalent ?
Because sulfur and oxygen are both non-metals, the formation of sulfur dioxide is a covalent bonding process. Two oxygen atoms encircle the sulfur atom in the middle.
Perchloric acid| Is It Super Acrid?
Is MgCl2 Ionic or Covalent?
Ionic Compounds| Properties, and Examples
SO2 Ionic or Covalent?
Is Ammonium Ion (NH4+) Polar or Nonpolar?
Acetyl Group| 9 Key Points-Easy Explanation
Torso'southward Preferred Source of Energy
Is NH3 Polar or Nonpolar?| Unproblematic Respond
So2 Molecular Geometry And Polarity,
Source: https://whatsinsight.org/so2-polar-or-nonpolar/
Posted by: clapperhavers.blogspot.com


0 Response to "So2 Molecular Geometry And Polarity"
Post a Comment